Our Latest News
Now Published – “The ReInvigorate Study—phrenic nerve-to-diaphragm stimulation for weaning from mechanical ventilation: a protocol for a randomized pivotal clinical trial” – link
August 2024 | News
Stimdia Medical Inc. Initiates FDA Approved IDE Pivotal Trial to Investigate Impact of Neuromuscular Stimulation on Mechanically Ventilated Patients – link
October 2023 | News
Stimdia Medical Announces Close of Series B Equity Financing Round with Proceeds to be Used to Complete the Company’s Pivotal Study – link
July 2022 | News
Stimdia Medical receives FDA breakthrough device designation for the pdSTIM™ System designed to facilitate weaning patients from mechanical ventilation – link
March 2021 | News
Results of PEPNS study published in Critical Care Medicine, official journal of the Society of Critical Care Medicine (SCCM) – link
May 2020 | News
Results of PEPNS study presented during the European Society of Intensive Care Medicine’s (ESICM) 32nd Annual Congress held in Berlin, Germany – link
September 2019 | News
Stimdia Medical reports completion of enrollment and follow-up for PEPNS study
February 2019 | News
Stimdia Medical reports successful completion of 30 day follow-up on first two patients enrolled into landmark PEPNS study
October 2018 | News
Stimdia Medical completes GLP chronic study for PEPNS System
March 2018 | News
Stimdia Medical receives 2nd Tranche for Series A1 Financing
March 2018 | News
Stimdia Medical receives 1st Tranche for Series A1 Financing
December 2017 | News
Stimdia Medical completes GLP acute phase study for PEPNS System
December 2017 | News
Stimdia Medical granted landmark foundational patent associated with electrical stimulation for preservation and restoration of diaphragm function
June 2017 | News
Stimdia Medical completes Series A Option 2x Financing
March 2017 | News
Andarta Medical changes name to Stimdia Medical
March 2016 | News
Andarta Medical closes Series A funding with world class investor syndicate Draper Triangle Ventures (Mr. Jay Katarinic), the University of Minnesota Discovery Capital Program (Mr. Russ Straate) and Industry Veteran Executive and Investor James Bullock
December 2015 | News
2023 – A Year of Milestones & Advancements
As we look back on the past year, we’re excited to share key milestones that have shaped our path and positioned us for a future characterized by innovation and expansion. Our unwavering dedication to excellence is highlighted by significant advancements in our pdSTIM System, the initiation of our pivotal IDE clinical study, strategic growth initiatives, and the addition of a renowned principal investigator to our team.
The milestones and achievements listed below (link to .pdf) serve as a solid foundation to propel us toward even greater success in 2024 and beyond. We extend our heartfelt gratitude to our dedicated team, partners, and supporters for their invaluable contributions to our efforts of empowering physicians and helping patients gain independence from mechanical ventilation. Thanks for being part of our journey!
Best,
Tim Miller
Stimdia Medical CEO
Completed Verification and Validation Testing of our Updated pdSTIM System
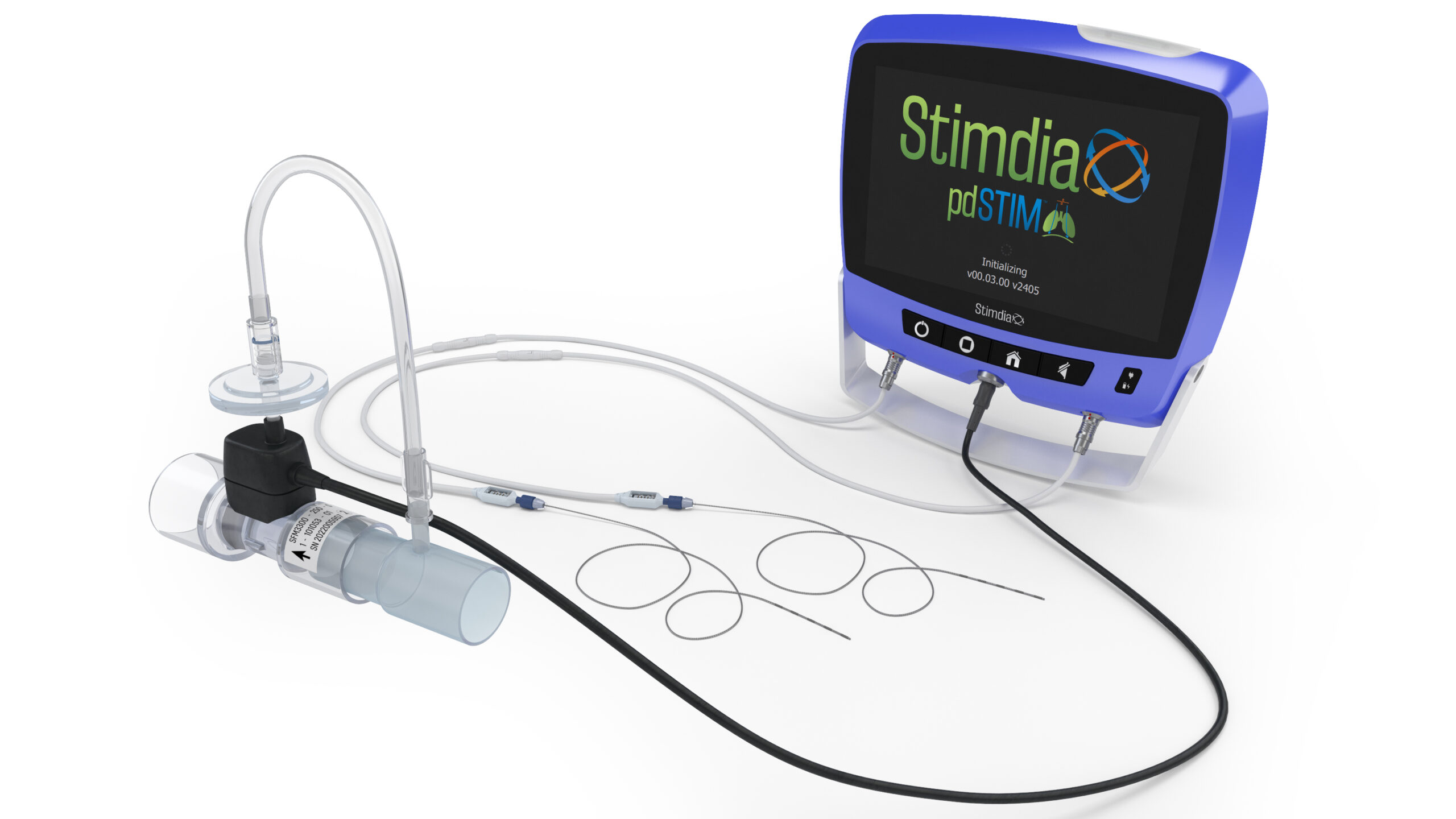 We evaluated and critiqued the performance of the pdSTIM device’s features to meet specifications for awakening and reconditioning the diaphragm. With the objective of equipping healthcare providers with a therapeutic solution that accelerates their patients’ transition from mechanical ventilation towards autonomous breathing, the System’s performance met or exceeded requirements and was robust enough for submissions to regulatory bodies.
We evaluated and critiqued the performance of the pdSTIM device’s features to meet specifications for awakening and reconditioning the diaphragm. With the objective of equipping healthcare providers with a therapeutic solution that accelerates their patients’ transition from mechanical ventilation towards autonomous breathing, the System’s performance met or exceeded requirements and was robust enough for submissions to regulatory bodies.
Obtained FDA Approval to Initiate our Pivotal Study, ReInvigorate
 The ReInvigorate Study is a randomized, controlled trial enrolling approximately 350 patients on mechanical ventilation in the ICU. Patients are randomly assigned to receive phrenic nerve stimulation (PNS) therapy using Stimdia’s pdSTIM System or standard of care. The study’s primary efficacy endpoint will evaluate the time to liberation from mechanical ventilation.
The ReInvigorate Study is a randomized, controlled trial enrolling approximately 350 patients on mechanical ventilation in the ICU. Patients are randomly assigned to receive phrenic nerve stimulation (PNS) therapy using Stimdia’s pdSTIM System or standard of care. The study’s primary efficacy endpoint will evaluate the time to liberation from mechanical ventilation.
Dr. Steven Conrad of LSU Health Sciences, Division of Critical Care Medicine, Joined Us as Our National Principal Investigator of The ReInvigorate Study
 “The pdSTIM System has the potential to introduce a novel approach to ICU patient management, assisting physicians, respiratory therapists, and nurses in expediting the liberation of patients from mechanical ventilation. I look forward to clinicians engaging in this therapy as part of the study and await the trial’s outcomes as it progresses toward completion.” – Dr. Steven Conrad
“The pdSTIM System has the potential to introduce a novel approach to ICU patient management, assisting physicians, respiratory therapists, and nurses in expediting the liberation of patients from mechanical ventilation. I look forward to clinicians engaging in this therapy as part of the study and await the trial’s outcomes as it progresses toward completion.” – Dr. Steven Conrad
Continued Clinical Trial Enrollment
 In 2023, we initiated enrollment and engaged with 28 out of the 35 total sites that will participate in The ReInvigorate Pivotal IDE Study. Stimdia is honored to collaborate with these clinical experts and applaud them for their pursuit of innovative technologies and procedures to enhance patient care while striving to reduce healthcare costs.
In 2023, we initiated enrollment and engaged with 28 out of the 35 total sites that will participate in The ReInvigorate Pivotal IDE Study. Stimdia is honored to collaborate with these clinical experts and applaud them for their pursuit of innovative technologies and procedures to enhance patient care while striving to reduce healthcare costs.
Submitted Three Articles for Presentation or Publication
 These articles detail our groundbreaking research and clinical findings related to our innovative pdSTIM System, highlighting its effectiveness and potential impact on patient care. These publications and presentations signify a significant step in establishing Stimdia Medical as a leader in the field and demonstrating the advancements we’re making in critical care medicine.
These articles detail our groundbreaking research and clinical findings related to our innovative pdSTIM System, highlighting its effectiveness and potential impact on patient care. These publications and presentations signify a significant step in establishing Stimdia Medical as a leader in the field and demonstrating the advancements we’re making in critical care medicine.
LSI – Emerging MedTech Summit
 Tim Miller, our CEO, presented at LSI USA ’23, showcasing the remarkable progress achieved with pdSTIM, significantly advancing critical care practices with neurostimulation technology, a much-needed therapy to help accelerate liberating patients from mechanical ventilators. Discussions underscored compelling value statements affirming the potential for pdSTIM to positively affect outcomes, safety, cost-efficiency, and operational efficacy.
Tim Miller, our CEO, presented at LSI USA ’23, showcasing the remarkable progress achieved with pdSTIM, significantly advancing critical care practices with neurostimulation technology, a much-needed therapy to help accelerate liberating patients from mechanical ventilators. Discussions underscored compelling value statements affirming the potential for pdSTIM to positively affect outcomes, safety, cost-efficiency, and operational efficacy.
Our Latest News
2023 – A Year of Milestones & Advancements – link
March 2024 | News
Stimdia Medical Inc. Initiates FDA Approved IDE Pivotal Trial to Investigate Impact of Neuromuscular Stimulation on Mechanically Ventilated Patients – link
October 2023 | News
Stimdia Medical Announces Close of Series B Equity Financing Round with Proceeds to be Used to Complete the Company’s Pivotal Study – link
July 2022 | News
Stimdia Medical receives FDA breakthrough device designation for the pdSTIM™ System designed to facilitate weaning patients from mechanical ventilation – link
March 2021 | News
Results of PEPNS study published in Critical Care Medicine, official journal of the Society of Critical Care Medicine (SCCM) – link
May 2020 | News
Results of PEPNS study presented during the European Society of Intensive Care Medicine’s (ESICM) 32nd Annual Congress held in Berlin, Germany – link
September 2019 | News
Stimdia Medical reports completion of enrollment and follow-up for PEPNS study
February 2019 | News
Stimdia Medical reports successful completion of 30 day follow-up on first two patients enrolled into landmark PEPNS study
October 2018 | News
Stimdia Medical completes GLP chronic study for PEPNS System
March 2018 | News
Stimdia Medical receives 2nd Tranche for Series A1 Financing
March 2018 | News
Stimdia Medical receives 1st Tranche for Series A1 Financing
December 2017 | News
Stimdia Medical completes GLP acute phase study for PEPNS System
December 2017 | News
Stimdia Medical granted landmark foundational patent associated with electrical stimulation for preservation and restoration of diaphragm function
June 2017 | News
Stimdia Medical completes Series A Option 2x Financing
March 2017 | News
Andarta Medical changes name to Stimdia Medical
March 2016 | News
Andarta Medical closes Series A funding with world class investor syndicate Draper Triangle Ventures (Mr. Jay Katarinic), the University of Minnesota Discovery Capital Program (Mr. Russ Straate) and Industry Veteran Executive and Investor James Bullock
December 2015 | News
2023 – A Year of Milestones & Advancements
As we look back on the past year, we’re excited to share key milestones that have shaped our path and positioned us for a future characterized by innovation and expansion. Our unwavering dedication to excellence is highlighted by significant advancements in our pdSTIM System, the initiation of our pivotal IDE clinical study, strategic growth initiatives, and the addition of a renowned principal investigator to our team.
The milestones and achievements listed below (link to .pdf) serve as a solid foundation to propel us toward even greater success in 2024 and beyond. We extend our heartfelt gratitude to our dedicated team, partners, and supporters for their invaluable contributions to our efforts of empowering physicians and helping patients gain independence from mechanical ventilation. Thanks for being part of our journey!
Best,
Tim Miller
Stimdia Medical CEO
Completed Verification and Validation Testing of our Updated pdSTIM System
We evaluated and critiqued the performance of the pdSTIM device’s features to meet specifications for awakening and reconditioning the diaphragm. With the objective of equipping healthcare providers with a therapeutic solution that accelerates their patients’ transition from mechanical ventilation towards autonomous breathing, the System’s performance met or exceeded requirements and was robust enough for submissions to regulatory bodies.
Obtained FDA Approval to Initiate our Pivotal Study, ReInvigorate
 The ReInvigorate Study is a randomized, controlled trial enrolling approximately 350 patients on mechanical ventilation in the ICU. Patients are randomly assigned to receive phrenic nerve stimulation (PNS) therapy using Stimdia’s pdSTIM System or standard of care. The study’s primary efficacy endpoint will evaluate the time to liberation from mechanical ventilation.
The ReInvigorate Study is a randomized, controlled trial enrolling approximately 350 patients on mechanical ventilation in the ICU. Patients are randomly assigned to receive phrenic nerve stimulation (PNS) therapy using Stimdia’s pdSTIM System or standard of care. The study’s primary efficacy endpoint will evaluate the time to liberation from mechanical ventilation.
Dr. Steven Conrad of LSU Health Sciences, Division of Critical Care Medicine, Joined Us as Our National Principal Investigator of The ReInvigorate Study
 “The pdSTIM System has the potential to introduce a novel approach to ICU patient management, assisting physicians, respiratory therapists, and nurses in expediting the liberation of patients from mechanical ventilation. I look forward to clinicians engaging in this therapy as part of the study and await the trial’s outcomes as it progresses toward completion.” – Dr. Steven Conrad
“The pdSTIM System has the potential to introduce a novel approach to ICU patient management, assisting physicians, respiratory therapists, and nurses in expediting the liberation of patients from mechanical ventilation. I look forward to clinicians engaging in this therapy as part of the study and await the trial’s outcomes as it progresses toward completion.” – Dr. Steven Conrad
Continued Clinical Trial Enrollment
 In 2023, we initiated enrollment and engaged with 28 out of the 35 total sites that will participate in The ReInvigorate Pivotal IDE Study. Stimdia is honored to collaborate with these clinical experts and applaud them for their pursuit of innovative technologies and procedures to enhance patient care while striving to reduce healthcare costs.
In 2023, we initiated enrollment and engaged with 28 out of the 35 total sites that will participate in The ReInvigorate Pivotal IDE Study. Stimdia is honored to collaborate with these clinical experts and applaud them for their pursuit of innovative technologies and procedures to enhance patient care while striving to reduce healthcare costs.
Submitted Three Articles for Presentation or Publication
 These articles detail our groundbreaking research and clinical findings related to our innovative pdSTIM System, highlighting its effectiveness and potential impact on patient care. These publications and presentations signify a significant step in establishing Stimdia Medical as a leader in the field and demonstrating the advancements we’re making in critical care medicine.
These articles detail our groundbreaking research and clinical findings related to our innovative pdSTIM System, highlighting its effectiveness and potential impact on patient care. These publications and presentations signify a significant step in establishing Stimdia Medical as a leader in the field and demonstrating the advancements we’re making in critical care medicine.
LSI – Emerging MedTech Summit
 Tim Miller, our CEO, presented at LSI USA ’23, showcasing the remarkable progress achieved with pdSTIM, significantly advancing critical care practices with neurostimulation technology, a much-needed therapy to help accelerate liberating patients from mechanical ventilators. Discussions underscored compelling value statements affirming the potential for pdSTIM to positively affect outcomes, safety, cost-efficiency, and operational efficacy.
Tim Miller, our CEO, presented at LSI USA ’23, showcasing the remarkable progress achieved with pdSTIM, significantly advancing critical care practices with neurostimulation technology, a much-needed therapy to help accelerate liberating patients from mechanical ventilators. Discussions underscored compelling value statements affirming the potential for pdSTIM to positively affect outcomes, safety, cost-efficiency, and operational efficacy.

